Projects
Project 1. How do T cells search for antigen in animals without lymph nodes?
In mammals, lymph nodes provide a dedicated site for diverse T cell populations to search for cognate antigen, and these organs are critical for adaptive immune function. Non-mammalian jawed vertebrates (fish, amphibians, reptiles, birds) maintain diverse T cells but lack lymph nodes, raising a basic immunology question: how do T cells search for antigen in animals lacking lymph nodes?
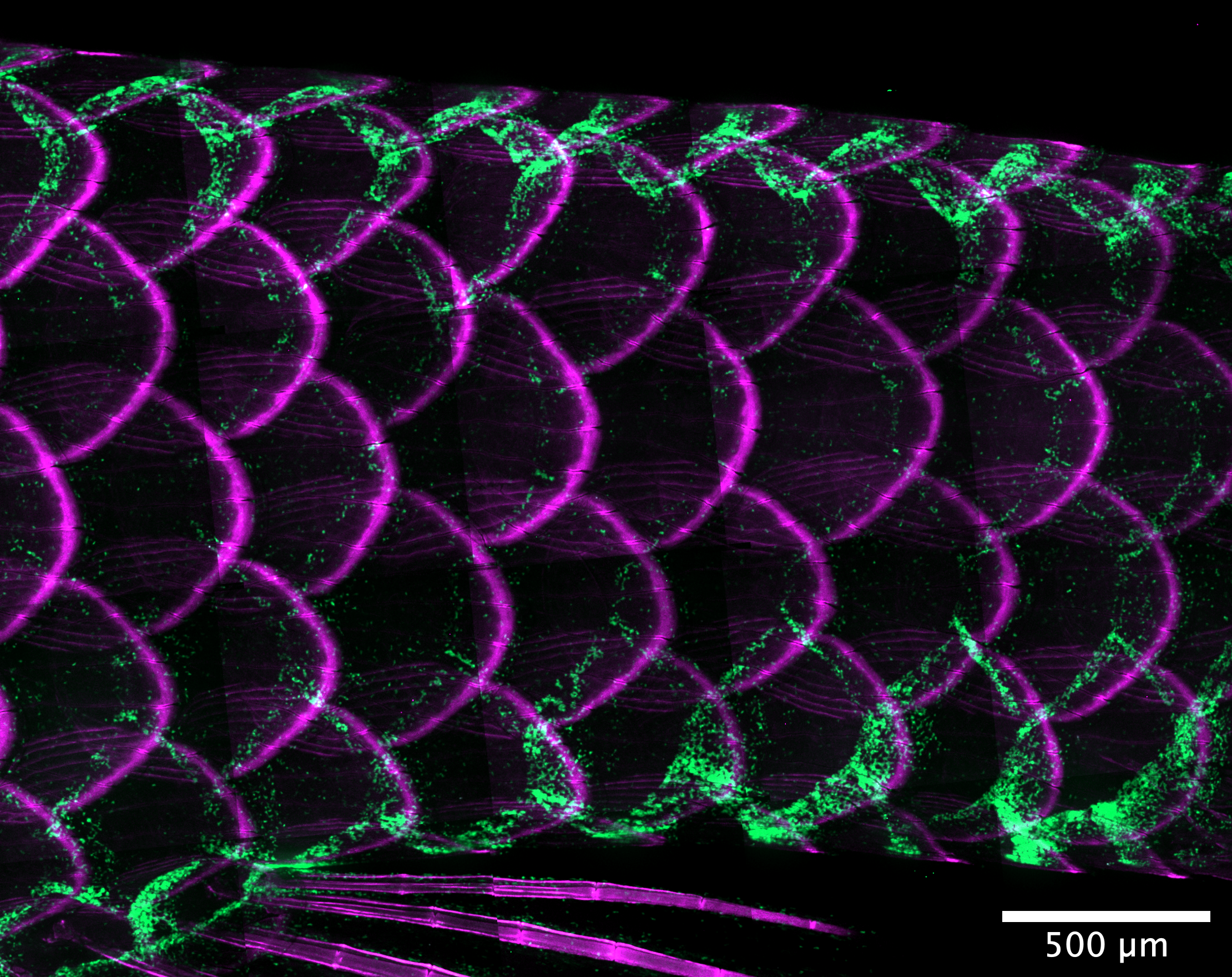
To address this question, I began studying T cell organization and motility in adult zebrafish in the lab of Anna Huttenlocher at the University of Wisconsin-Madison. Here, I found that T cells organize into a body-spanning lymphoid tissue interleaved between the scales (Fig). Despite not being previously characterized, the TLN is the largest lymphoid tissue in zebrafish, harboring ~50% of all host T cells. My colleagues and I found that the TLN enables T cells to traffic through the host in a coordinated ventral-to-dorsal loop, enabling whole-body antigen search in the absence of a lymph node system. The immunological function and genetic regulation of the TLN will be a major focus of my future research program.
Project 2. What is the molecular basis for T cell collective migration?
I found that in the TLN, T cells can form head-to-tail interactions and migrate collectively as multicellular streams. This mode of cooperative multicellular motility was recently found to be conserved in mammals, although the physiological function of this behavior remains unclear. A major focus of my laboratory will be determining the molecular nature of the T cell head-to-tail interaction and how disrupting this multicellular streaming affects motility and immune function in vivo.
Project 3. Do amoeboid cells tailor their migration strategy to their local tissue environment in vivo?
Depending on their environment, amoeboid cells like leukocytes can migrate via distinct mechanisms (i.e., pseudopods, lamellipodia, intermittent blebs, stable blebs) in vitro, but why cells maintain this migratory plasticity remains unclear. One hypothesis is that cells can toggle between these different modes of migration because it enables them to navigate the different tissue environments that exist in vivo. To test this hypothesis, I utilized larval zebrafish to image T cell f-actin dynamics as cells migrated in discrete tissue environments in vivo and found that in most tissues T cells migrate via pseudopods but utilize the stable bleb mode of migration, characterized by a large cytoplasmic leading edge and retrograde actin flow, specifically with in the epidermis. This mode of migration is commonly observed for cells placed under planar confinement in vitro and we find that epidermal T cells are under a similar kind of planar confinement in vivo sandwiched between keratinocyte layers. My laboratory will continue to study how confinement and adhesion regulate amoeboid motility in cellularly dense environments like the epidermis in vivo.